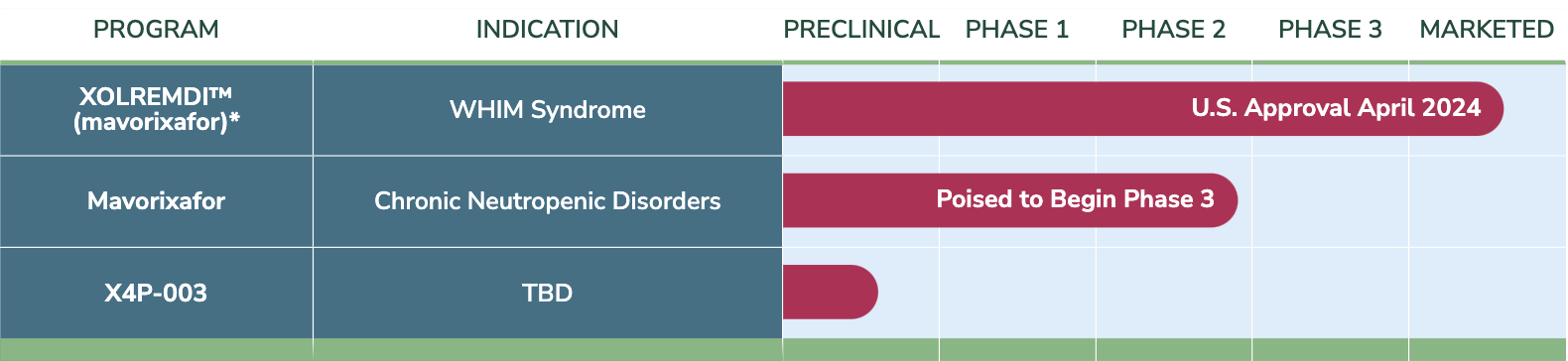
Advancing Candidates
Leveraging our unparalleled expertise in diseases of the immune system and CXCR4 biology, we aim to bring innovative treatments to people with rare diseases of the immune system.
Our Focus on CXCR4
CXCR4, or C-X-C receptor type 4, and its ligand, CXCL12, have been shown to play a key role in regulating the mobilization of white blood cells, including neutrophils, lymphocytes, and monocytes, from the bone marrow into peripheral circulation. Because inhibition of the CXCR4 receptor has been shown in the clinic to elevate levels of circulating white blood cells, we believe that therapies targeting the CXCR4/CXCL12 pathway hold the potential to benefit patients across a variety of diseases of the immune system.
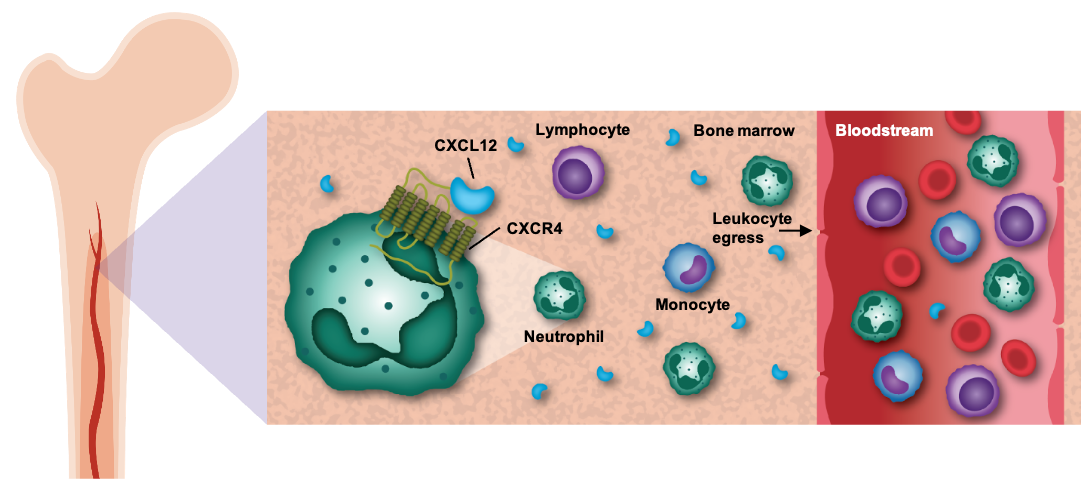
The CXCR4 pathway regulates the mobilization of white blood cells from the bone marrow to the peripheral blood
About Mavorixafor
Our deep understanding of the biology of the CXCR4 pathway has enabled us to advance mavorixafor, a selective CXCR4 antagonist, through clinical development to commercialization in its first indication. We are exploring the use of mavorixafor in additional patient populations as well, including those with certain chronic neutropenic disorders.
XOLREMDI™
(mavorixafor)*
Mavorixafor
X4P-003
*Please read the full Prescribing Information
*Please read the full Prescribing Information
Advancing Mavorixafor in Chronic Neutropenic Disorders
- Chronic neutropenia is a rare blood condition defined by a decrease in blood neutrophil counts lasting more than three months; people with chronic neutropenia are at higher risk of developing infections and certain cancers and having a reduced quality of life.
- There has been little innovation for people with chronic neutropenia over the past 30 years; the only medicine approved to treat those living with severe chronic neutropenia is granulocyte colony-stimulating factor (G-CSF), an injectable therapy.
- Following successful completion of Phase 1b and Phase 2 clinical trials exploring the use of once-daily oral mavorixafor in the treatment of certain chronic neutropenic disorders, we are poised to begin a pivotal, global Phase 3 clinical trial.
- The Phase 3 4WARD trial (NCT06056297) aims to evaluate the efficacy, safety, and tolerability of oral once-daily mavorixafor (with or without G-CSF) in people with congenital or acquired primary autoimmune and idiopathic chronic neutropenia who are experiencing recurrent and/or serious infections. For more information, please visit 4WARDstudy.com.
Unparalleled Expertise in CXCR4 and Immune System Biology
In addition to our headquarters in Boston, Massachusetts, we have an established research center of excellence in Vienna, Austria. Here, our scientists not only focus on new drug discovery and deepening the understanding of the role of the CXCR4/CXCL12 axis in maintaining immunity, but also on examining the genetic causes of certain chronic neutropenic disorders.